DIESSE is an Italian company with an integrated and entirely inhouse production of in vitro diagnostic systems. Its headquarters are in Siena.
Since its foundation in 1980, the company has developed, produced and marketed innovative diagnostic systems mainly in the area of immunochemistry and automatic measurement of ESR. The company features a worldwide presence in over 100 countries, a manufacturing plant and a research centre in which the automated production of diagnostic tests, analytical instruments and software meets Italian design and cutting-edge technology, branding DIESSE as "Diagnostics for the immune system"
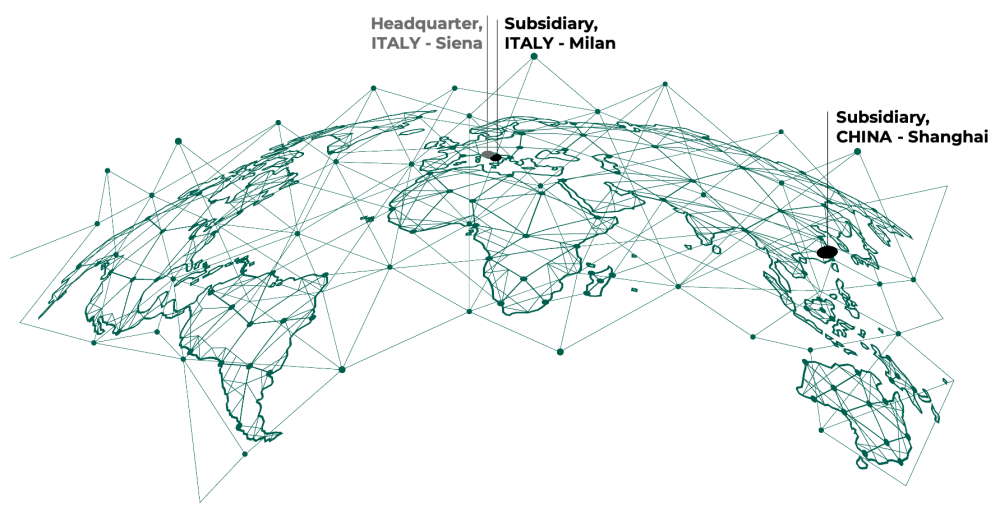

We use our history and our skills in everyday work, integrating advanced research, Made in Italy production and global marketing, for the evolution of our diagnostics systems towards the medicine of future in a sustainable, ethical and transparent way.

- Ethics & sustainability
- Transparency
- Sense of belonging
- Research & innovation
- Sharing
- Expertise
- Tenacity
- People center
